
research and publications
Clinical Trial
Effecti-Cal®, a Novel Formulation of Calcium, Vitamin D and Minerals for Prevention of Postmenopausal Bone Loss
- 97 subjects- postmenopausal women
- 34 weeks
- Double blind, randomized comparative clinical trial
- Dosage- 150 mg twice a day
Effecti-Cal® Phase IV Results
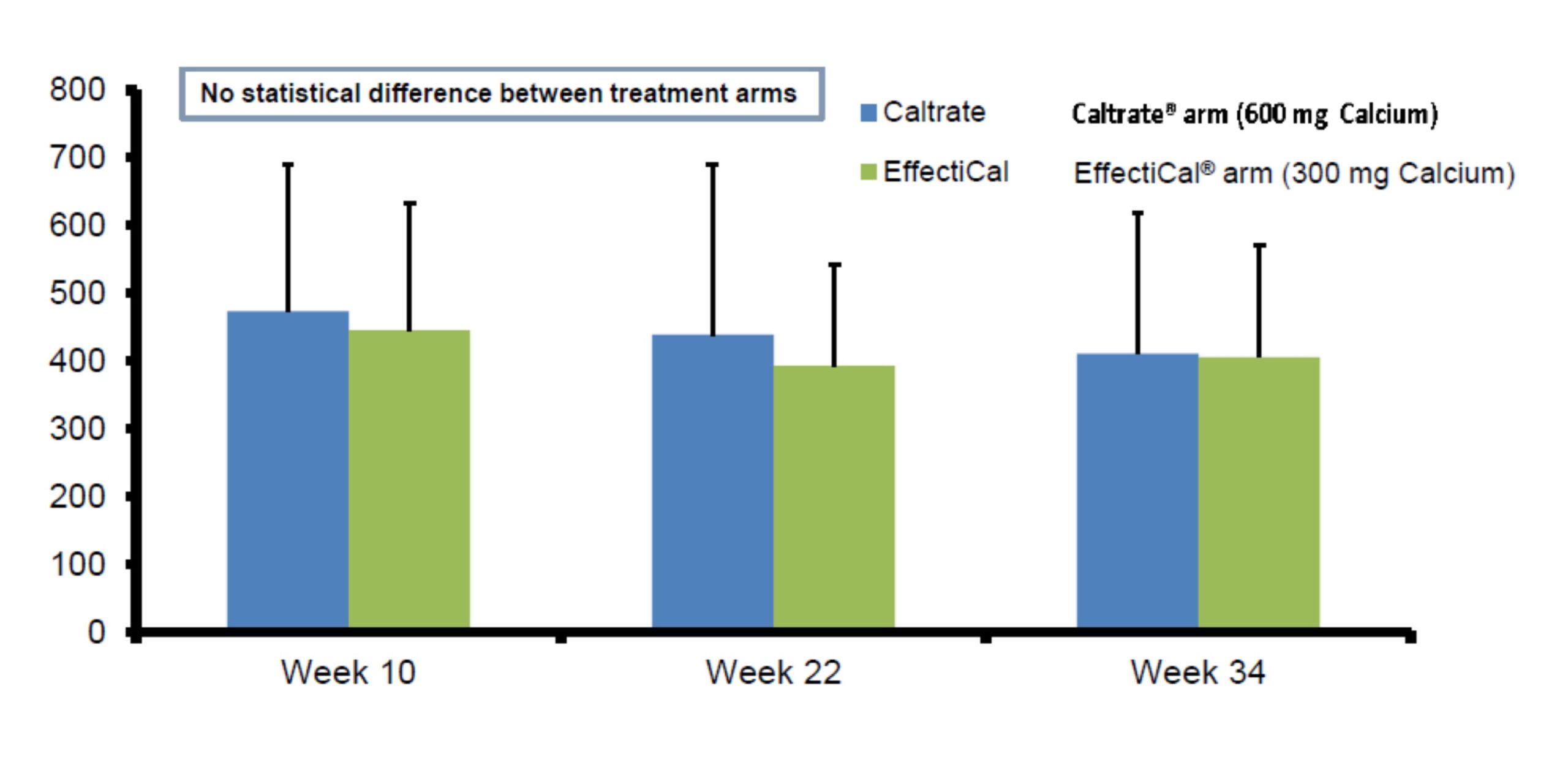
Plasma CTx (bone resorption) values were similar with EffectiCal ® compared to Caltrate ® (calcium carbonate) + Vitamin D3
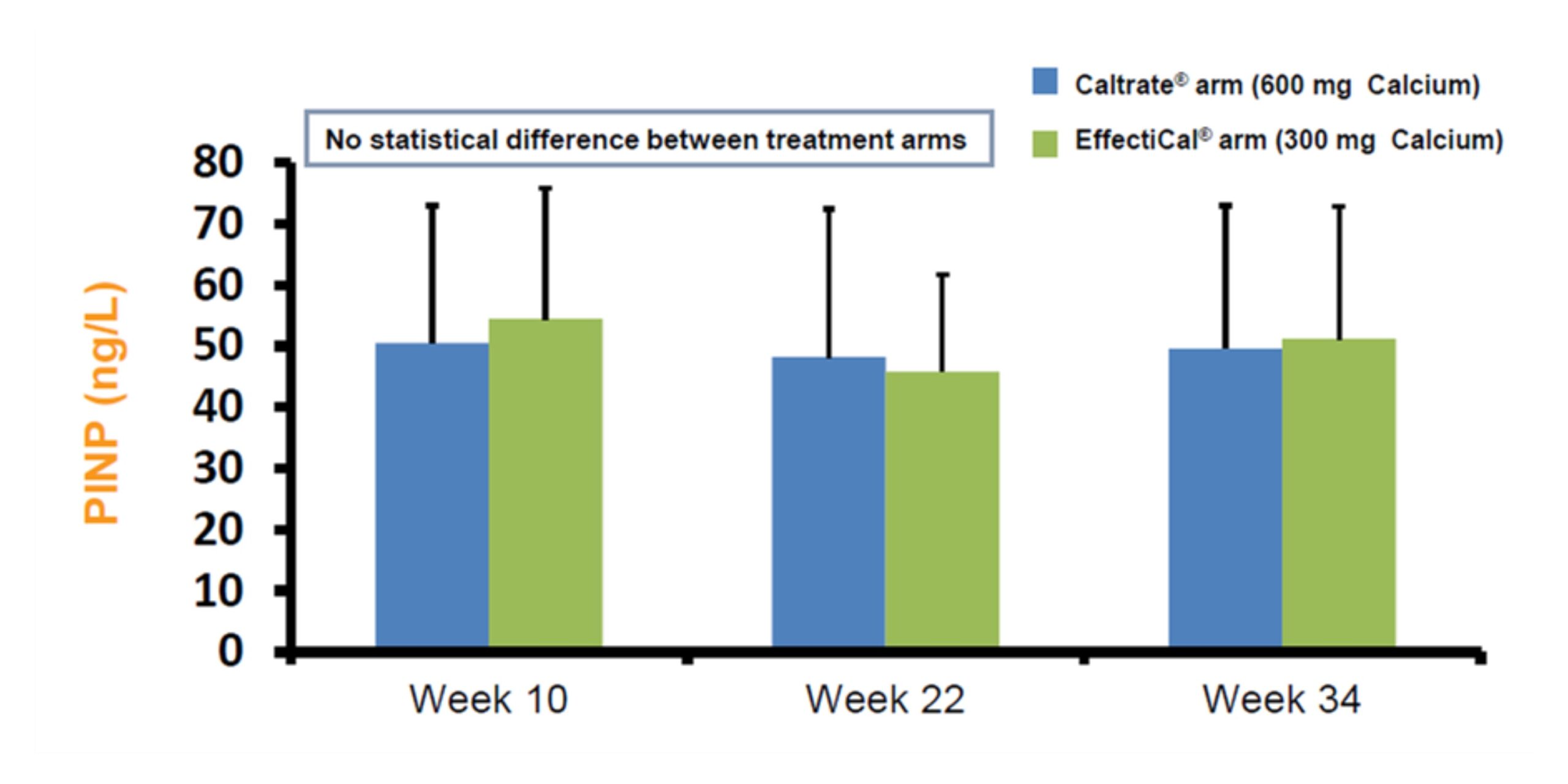
Plasma PINP (bone formation) values were similar with EffectiCal ® compared to Caltrate®(calcium carbonate) + Vitamin D 3
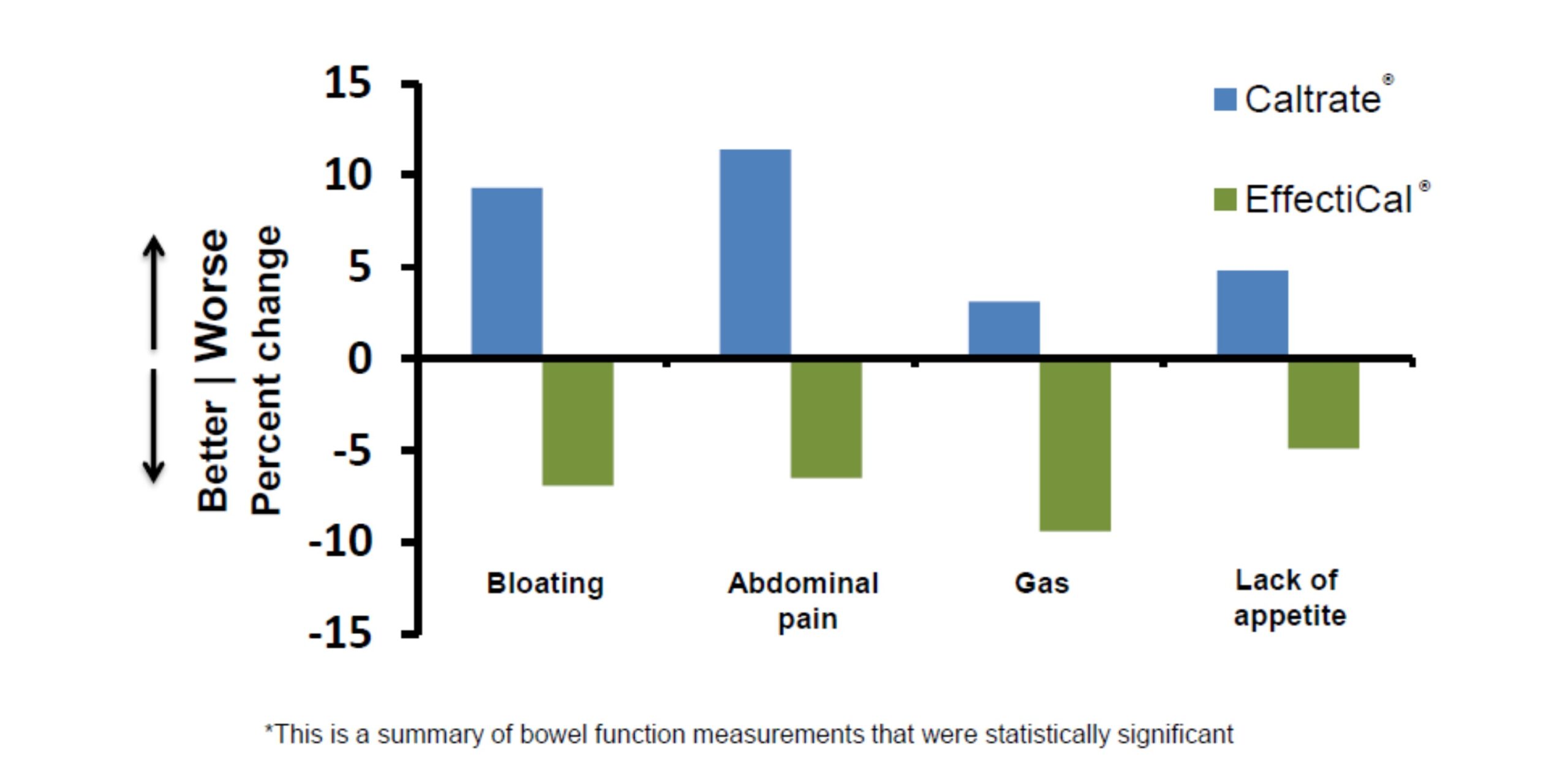
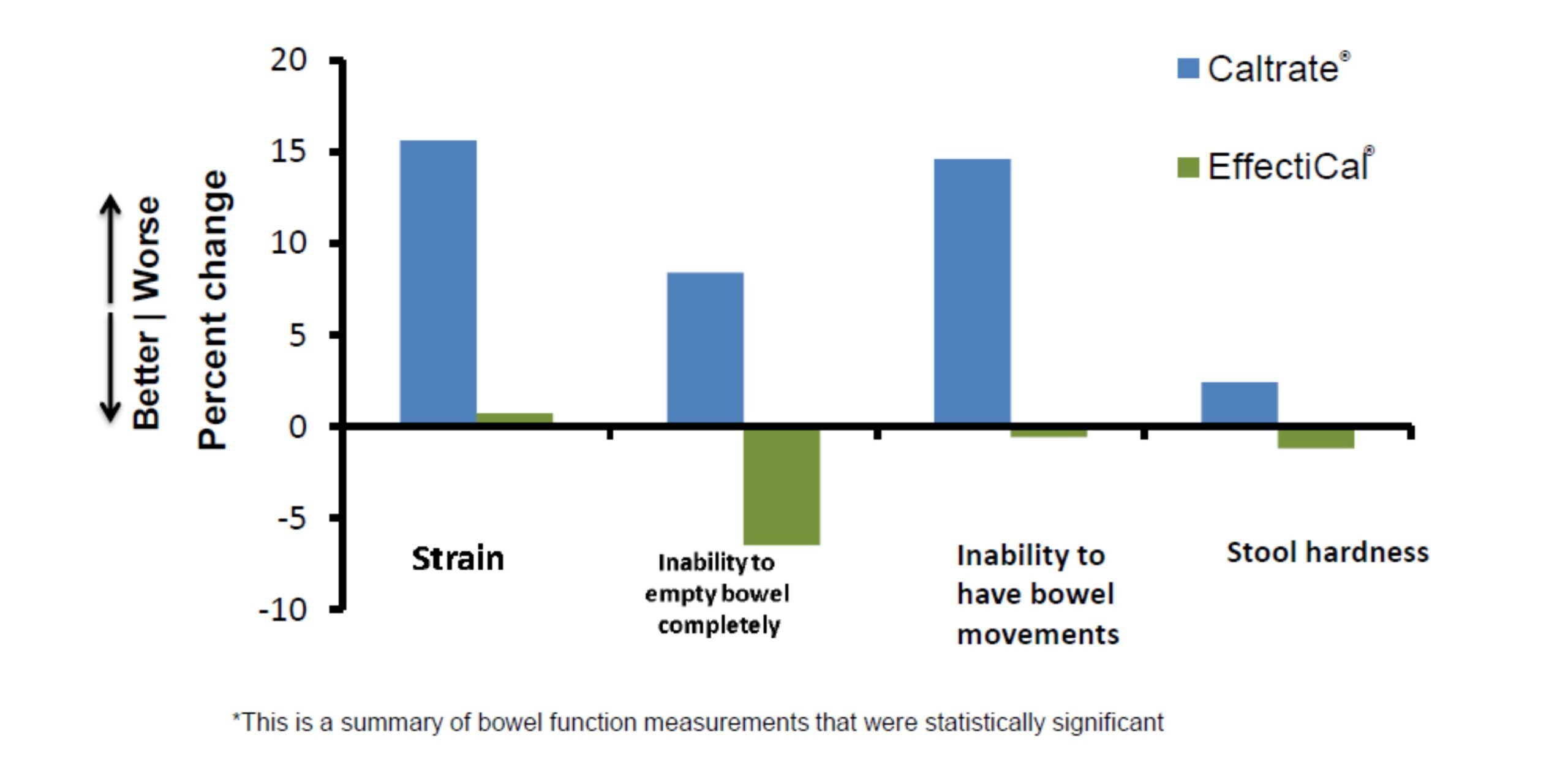
Summary of Clinical Trial Results
- Effecti-Cal® had better activity per dose of calcium than Caltrate®.
- Effecti-Cal® (300mg of calcium) and vitamin D3 was no different than Caltrate® (600mg calcium) and vitamin D3 in measurements of bone resorption (CTx) and bone formation (PINP).
- Effecti-Cal® has a better gastrointestinal profile versus Caltrate®:
- Less constipation.
- Less strain and improved bowel emptying.
- Less bloating, abdominal pain and gas.
- Less lack of appetite.
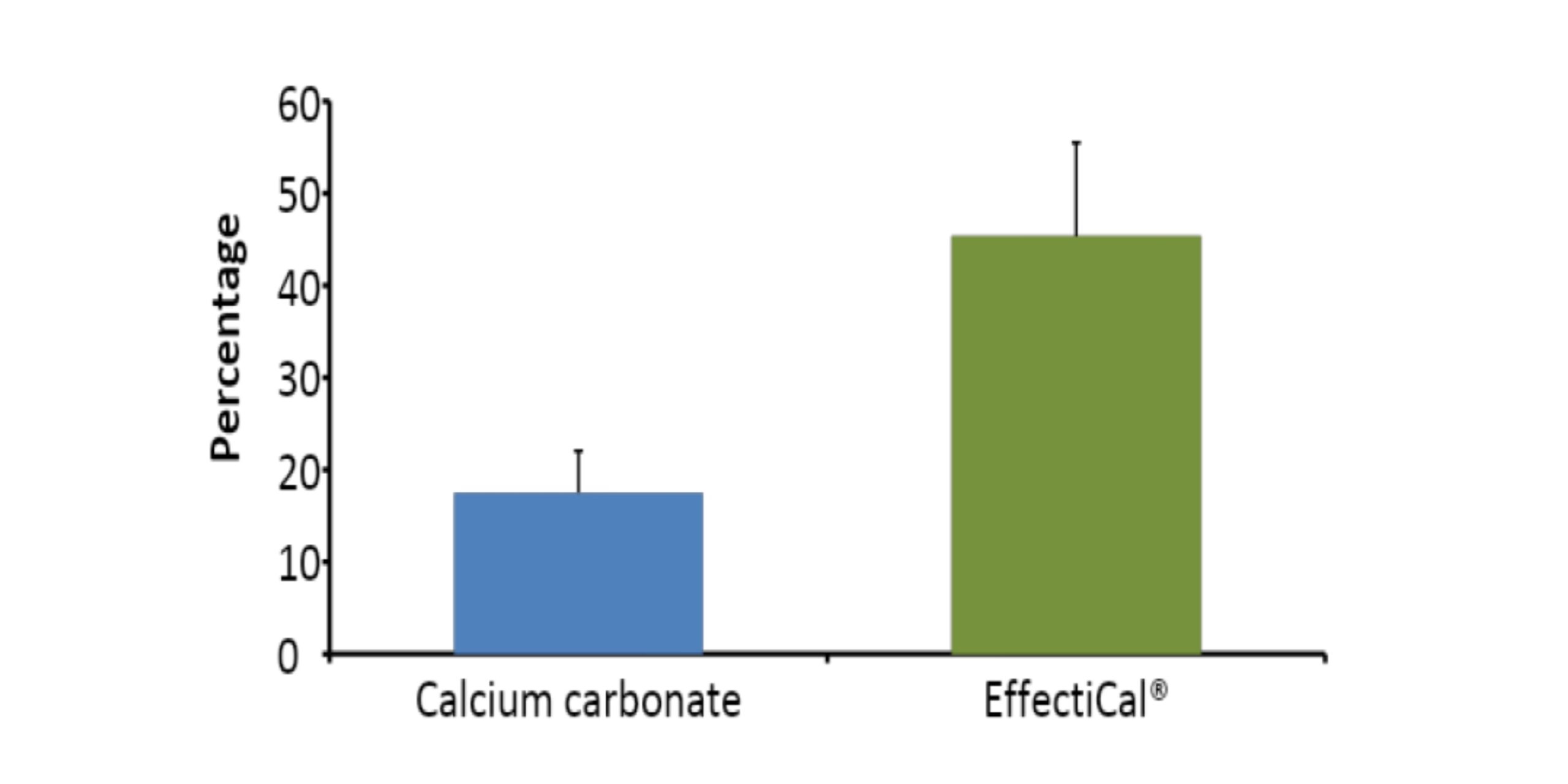
Calcium retention after an oral dose of Effectical was approximately 45%. For calcium carbonate it was approximately 18%


